A study published in Cancer Cell and funded by the Ministry of Health and AIRC sketches a new profile of pancreatic tumor cells by associating specific biological characteristics with different morphological aspects of the cells. Led by a team of researchers from the European Institute of Oncology (IEO) coordinated by our IPCC member Giuseppe Diaferia and Gioacchino Natoli, the research reveals how various types of tumor cells coexist within each tumor and how this heterogeneity impacts prognosis.
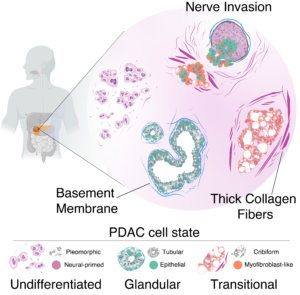 “It’s like we’re fighting against multiple opponents at the same time,” explained Pierluigi Di Chiaro, lead author of the article. “Identifying different tumor niches that grow simultaneously within the pancreas will allow the development of treatments capable of intercepting all the populations of neoplastic cells that make up each individual tumor. These niches are able to remodel and adapt to the surrounding environment, and discovering these characteristics lays the groundwork for defining new strategies.”
“It’s like we’re fighting against multiple opponents at the same time,” explained Pierluigi Di Chiaro, lead author of the article. “Identifying different tumor niches that grow simultaneously within the pancreas will allow the development of treatments capable of intercepting all the populations of neoplastic cells that make up each individual tumor. These niches are able to remodel and adapt to the surrounding environment, and discovering these characteristics lays the groundwork for defining new strategies.”
One of the main findings of the study concerns the invasion of nerves by pancreatic cancer cells, one of the main causes of the unbearable pain associated with this disease. “Nerve invasion represents a real escape route that tumor cells use to spread throughout the body without encountering obstacles,” explains Lucia Nacci, co-author of the study. The researchers observed how the activation of certain genes is correlated with the ability to invade nerves, revealing potential mechanisms to prevent tumor spread.
“The study highlights how it is possible to give a face to the different tumor cells associated with different functional properties and use this profile to easily identify their presence in each individual patient”. This demonstration, explains Giuseppe Diaferia, lays the foundation for the development of artificial intelligence approaches capable of predicting, already in routine histological examinations, the composition of the tumor and guiding the physician in choosing the most suitable combination of drugs for each patient. “This approach could greatly facilitate diagnosis and treatment effectiveness”.
“Understanding the heterogeneity of pancreatic cancer is crucial for developing effective therapeutic strategies,” concluded Natoli. “Although this work is the result of years of technological optimizations and conceptual advancements, it is only a starting point for new research that can pave the way for personalized therapeutic approaches that will finally offer new hope to patients facing this devastating disease.”
Published in Cancer Cell, Marzo 2024: “Mapping functional to morphological variation reveals the basis of regional extracellular matrix subversion and nerve invasion in pancreatic cancer“. Cancer Cell, 2024 Mar; https://doi.org/10.1016/j.ccell.2024.02.017
The pre-clinical study published by the prestigious international journal Gut demonstrates how PI3K-C2γ plays a key role in the development of one of the most aggressive cancers.
A new preclinical study, carried out in the “Guido Tarone” Center for Molecular Biotechnology of the University of Turin, made it possible to discover a new targeted therapy for a subgroup of patients affected by pancreatic ductal adenocarcinoma. The research group of the Department of Molecular Biotechnology and Health Sciences, led by Prof. Miriam Martini and Prof. Emilio Hirsch, has shown that the PI3K-C2γ protein plays a key role in the development of pancreatic cancer. This study has made it possible to shed light on the mechanisms of development of this tumor and might, in the future, maximize the effecacy of the current therapeutic options for one of the most aggressive tumors.
In the world, the number of new cases of pancreatic cancer is increasing of about 1% and the 5-year survival rate is less than 10%. Pancreatic cancer is expected to become the second leading cause of cancer death by 2030. The severity and lack of effective treatments need efforts to search for new therapies and markers that help in choosing the most effective drug.
To grow, cancer cells need nutrients and energy sources. The aggressiveness of pancreatic cancer is due to the ability to adapt to adverse conditions, such as the paucity of nutrients and energy sources, which are exploited by cells to survive. Recently, drugs have been developed that prevent the use of these nutrients, such as glutamine.
PI3K-C2γ controls the intracellular signaling pathway of mTOR, which regulates cell metabolism and growth, and affects the use of glutamine to promote tumor progression. In pancreatic cancer, the PI3K-C2 protein is lost in about 30% of patients, who develop a more aggressive form of the disease
Dr. Maria Chiara De Santis, first author of the study published in the prestigious international journal Gut, has shown that the loss of PI3K-C2γ accelerates tumor development, but makes it more sensitive to drugs that affect mTOR and to the use of glutamine.
The study led by the UniTo scientists was the result of intense collaborative work with groups in the Italian and international territory and the Italian Pancreatic Cancer Community (I-PCC), including those of Prof. Francesco Novelli, Prof. Paola Cappello and Prof. Paolo Ettore Porporato (University of Turin), Prof. Andrea Morandi (University of Florence), Prof. Vincenzo Corbo and Prof. Aldo Scarpa (University of Verona), Prof. Gianluca Sala and Prof. Rossano Lattanzio (University of Chieti) and Prof. Elisa Giovannetti (University of Amsterdam and Pisana Foundation for Science).
Here the link to the paper: Lysosomal lipid switch sensitises to nutrient deprivation and mTOR targeting in pancreatic cancer, Gut, https://gut.bmj.com/content/72/2/360
Congratulations to one of our I-PCC member founders Dr. Loretta L. del Mercato (Institute of Nanotechnology of the National Research Council of Lecce) for her collaboration in a recent study with the Biofisika Institute (Spain), the Ikerbasque Foundation (Spain), the Italian Institute for Genomic Medicine – IIGM, the Turin Polytechnic, the University of Salento (Lecce), the ‘Giovanni Paolo II’ Ircss Cancer Institute of Bari and Technopole for precision medicine of the Apulia Region.
The researchers developed a platform that reconstructs the ecosystem underlying the development of tumors, starting from the analysis of cell metabolism. “We developed an in vitro microenvironment similar to the natural one for the development of pancreatic cancer cells, creating nanofibers containing optical sensors that mimic the structure of the extracellular matrix, the part of the tissues in which there are no cells. These membranes allow to reconstruct, with a high spatial and temporal resolution, the proton fluxes and the exchange networks between cells within a heterogeneous cell population: the differences between single cells, in fact, strongly influence the collective behavior of the systems and, consequently, they can affect the effectiveness of medical treatments” explains Loretta L. del Mercato.
This study paves the way for non-invasive, inexpensive, real-time analysis of single cell metabolism and identification of new drug combinations that could represent a breakthrough in the treatment of pancreatic cancer.
Here the link to the paper: Probing Single-Cell Fermentation Fluxes and Exchange Networks via pH-Sensing Hybrid Nanofibers, ACS Nano, https://doi.org/10.1021/acsnano.2c06114.
A press release is available at: https://www.cnr.it/it/comunicato-stampa/11662/pancreas-ricostruito-l-ecosistema-cellulare-alla-base-dello-sviluppo-dei-tumori
Pancreatic cancer has limited treatment options due to the drug-resistant nature of this tumor. However, the mechanisms underlying this phenomenon are still largely unknown and the identification of new factors contributing to the pancreatic cancer chemoresistance is urgently warranted.
To consolidate existing networks of pancreatic cancer research, as well as to set up new partnerships and collaborations between high-level pancreatic cancer research institutions, the European multi-stakeholder platform Pancreatic Cancer Europe has recently promoted a Short Stay Scientific Award (S3A) to support young investigators exchange between European laboratories.
This year this S3A grant has been awarded to Mjriam Capula, researcher at Scuola Superiore Sant’Anna in Pisa and Fondazione Pisana per la Ricerca (FPS), in order perform a research internship at the CCA on the role of microbiome in pancreatic cancer chemoresistance.
Tumor microbiome has been recently recognized among the hallmarks of cancer, and tumor-associated microbiota has recently emerged for its potential role in mediating resistance to several anti-cancer agents, including drugs commonly used for pancreatic cancer treatment.
Expression of the long-variant of the enzyme cytidine deaminase by several intratumor bacteria was shown to be responsible for the resistance to the commonly used anticancer drug gemcitabine. Beside this, other bacteria can affects drug activity via autophagy modulation, suggesting that chemoresistance conferred by bacteria might be induced by multiple mechanisms. To date these mechanisms have been studied in a limited number of preclinical models and the knowledge of specific factors responsible for this phenomenon in pancreatic cancer is still limited. Thus, the purpose of this study is to characterize the ability of selected bacterial species to induce chemoresistance in new models of pancreatic cancer and to identify the molecular mechanisms underlying these effects.
This research is supervised by Prof. Luca Morelli at the University of Pisa, while Prof. Elisa Giovannetti and Prof. Geert Kazemier are leading studies on microbiome in pancreatic cancer at FPS and Cancer Center Amsterdam. This project is also a collaborative project with Dr. Dongmei Deng from ACTA.
“My experience in Amsterdam gave me a greater understanding of different work perspectives, and has taught me that multidisciplinary teamwork and collaborations with institutions of excellence are essential to improve our knowledge of cancer biology and hopefully enable rapid translation of new discoveries into better therapeutic interventions for cancer patients.” – Dr. Mjriam Capula.
More info here: https://youtu.be/BEz_3lLYgcM
Hypoxia is a common feature of solid tumors, due to the rapid growth of the tumor that exceeds the oxygen supply, and impaired vascular network for the formation of abnormal blood vessels supplying the tumor. During tumor development and progression, this hypoxic microenvironment can activate angiogenesis, thus increasing tumor survival, invasiveness, distant metastasis and hamper the therapeutic response.
Dr. Stefania Forciniti, INTERCELLMED-ERC research fellow at the CNR-Nanotec of Lecce (IT) in the group of Dr. Loretta L. del Mercato, won a 3-month short-term mobility funded by AIRC at the Research Group of 3B, I3Bs – Research Institute on Biomaterials, Biodegradables and Biomimicry, University of Minho (PG), in the group of Prof. Miguel Oliveira for the project “Characterization and validation of oxygen sensing platforms for monitoring in real-time in vitro tumor hypoxia”. Dr. Stefania Forciniti will apply ratiometric optical oxygen sensors, developed at CNR-Nanotec, in patient-derived in vitro cancer models to monitor oxygen concentrations in real time and to study how different tumor cells respond to pharmacological stress.
These studies are oriented towards precision medicine which leads to the choice of the right personalized therapy for each individual patient.
A recent review out this month in the prestigious journal Nature Reviews Drug Discovery, discussing “Macrophages as tools and targets in cancer therapy”. The group of authors, coordinated by Professor Alberto Mantovani, at Humanitas Research Hospital and featuring one of our I-PCC members, Dr. Federica Marchesi, summarizes current strategies aimed at therapeutically targeting macrophages in the tumor microenvironment.
The landscape of anticancer strategies has been profoundly modified in the last few years, with continuous efforts aimed at complementing current therapeutic options directed against tumor cells with new approaches targeting immune cells infiltrating tumor tissues. These new approaches, collectively named Immunotherapies, have the potential to fight cancer from within, stimulating or reprogramming the patient’s immune system to attack the tumor. Among immunotherapies, the best known are probably checkpoint inhibitors, which have shown durable responses in some tumor types, such as lung cancer and melanoma. In this review, Dr. Federica Marchesi and colleagues focus on macrophages, abundant immune cells in human cancer tissues, mostly engaged in pro-tumor responses, but ideal cells to reprogrammed due to their peculiar versatility. Macrophage-centered therapeutic strategies have the potential to complement and synergize with currently available tools in the oncology armamentarium.
As we learn day by day, pancreatic cancer is an aggressive tumor, characterized by a compromised immune system that does little to control the growth of malignant cells. The attention is now focused on the possibility that new immune-based approaches, such as the ones targeting macrophages may change this scenario.
Here the link to the paper: https://rdcu.be/c7inj
Mantovani, A., Allavena, P., Marchesi, F. et al. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov 21, 799–820 (2022). https://doi.org/10.1038/s41573-022-00520-5
Congratulations to Dr. Alessandro Carrer, one of the founding members of I-PCC, for receiving the prestigious #WCRF grant to study “Whether the excessive ingestion of fructose, a widely popular natural sweetener added to most industrial beverages, can put you at risk of pancreatic cancer”. If you want to learn more about that please visit: https://www.fondbiomed.it/cancro-e-bevande-zuccherate-wcrf-finanzia-la-ricerca-di-alessandro-carrer/?fbclid=IwAR0cdDWtkJtrDM9ahchB-0I-i5WvZEaShTbFhIabnppcAeM-noBJ-f7yNoQ and https://www.wcrf.org/researchwefund/do-sugary-drinks-increase-pancreatic-cancer-risk/
Congratulations to I-PCC members Dr. Miriam Martini and Dr. Elisa Giovannetti for their collaboration in a recent study with Dr. Andrea Costamagna and Professors Emilio Hirsch and Sara Cabodi from the Center for Molecular Biotechnology of the University of Turin, Italy, Fondazione Pisana per la Scienza, Pisa and Amsterdam University Medical Centers, Amsterdam, The Netherlands.
The researchers analyzed human pancreatic tumor samples and mouse models of pancreatic cancer of increasing aggressiveness in order to unravel the relationship between p130Cas and pancreatic tumorigenesis. The study demonstrated that p130Cas acts downstream of Kras to increase PI3K-AKT signaling required for acino-ductal metaplasia and subsequent tumor development. This research also contributes to a better understanding of pancreatic cancer prognosis. The discovery of key proteins that regulate the early stages of tumorigenesis is fundamental to fully understand this pathology and to identify new potential molecular markers useful in diagnostics and therapy.
Here the link to the paper: https://pubmed.ncbi.nlm.nih.gov/34922945/
A press release is available at: https://www.amsterdamumc.org/en/research/institutes/cancer-center-amsterdam/news/a-new-essential-factor-for-the-onset-of-pancreatic-cancer.htm
Congratulations to Dr. Paola Cappello one of the founding members of @I-PCC for organizing the 2nd International Electronic Conference on Cancers (IECC 2022), entitled “Tumor Microenvironment Heterogeneity in Cancer Progression: Challenge or Opportunity” https://iecc2022.sciforum.net/ which will be held from 14 to 16 February in virtual mode.
The focus of this conference is the translation of basic science understanding of the tumor microenvironment and its dysregulation in cancer to its therapeutic exploitation. Topics of interest include:
Models to study tumor heterogeneity and clonality, targeted therapies exploiting tumor heterogeneity and components of the microenvironment, strategies to unleash the anti-tumor response and overcome intrinsic tumor resistance to therapies, clinical studies with tumor heterogeneity and tumor microenvironment-targeted therapies.
During the Conference many of @I-PCC members will talk about their research projects.
Dr. Paola Cappello will talk about “IL17A drives stromal reaction in pancreatic cancer toward the immunosuppression: a chance to re-shape the tumor microenvironment”
Dr. Federica Marchesi will talk about “Macrophages in human cancer: clinical relevance and therapeutic potential”
Dr. Vincenzo Corbo will talk about “Subtype-specific stroma in pancreatic cancer”
Dr. Donatella Delle Cave postdoctoral fellow in Dr. Enza Lonardo’s laboratory will talk about “Unraveling the contribution of L1low cancer cell-derived collagen in chemoresistance and metastasis of pancreatic cancer”
Dr. Riccardo Rizzo researcher in Loretta del Mercato’s laboratory will talk about “3D sensing scaffold for extracellular pH mapping at single cell level: A Pre-Clinical Model to Study Cancer Heterogeneity”
Dr. Stefania Forciniti postdoctoral fellow in Dr. Loretta del Mercato’s laboratory will talk about “Emerging 3D in vitro pancreatic cancer models for tumor heterogeneity studies and personalized therapies”
The Foundation has launched the phase 2 Nab-PIPAC study, which will explore the results of administering the chemotherapeutic agent Nab-paclitaxel intraperitoneally through pressurized aerosol (PIPAC) on peritoneal metastases from pancreatic cancer. The experimental treatment will be performed laparoscopically in a series of sessions over a six-month period, in conjunction with standard therapy.
“This is a phase 2 study,” explains Dr. Andrea Di Giorgio, co-Principal Investigator of the study and head of the Integrated Treatments of Advanced Peritoneal Carcinomatosis, Peritoneal and Retroperitoneal Surgery at the Policlinico Gemelli. “The real innovation is the intraperitoneal use of Nab-paclitaxel through PIPAC, a drug already used for systemic treatment and active against pancreatic cancer. The study, which will be conducted at the Gemelli, will enroll a total of 38 patients. In this regard,” Dr. Di Giorgio continues, “we invite patients with peritoneal metastases from pancreatic cancer to discuss this study with their oncologist and contact us ([email protected]) if interested in participating.”
“Nab-PIPAC is a study,” emphasizes Prof. Giampaolo Tortora, director of the Comprehensive Cancer Center and co-Principal Investigator of the study, “that should provide solid evidence to understand how this technologically innovative approach can contribute to improving the activity of chemotherapeutic drugs currently used to treat pancreatic tumors.”
This study also involves the I-PCC network directly – the Italian communit
y that brings together scientists in the field of basic and translational research on pancreatic cancer – which aims to identify new markers and vulnerabilities of the tumor. This community is supported and promoted by the NADIA VALSECCHI ONLUS FOUNDATION (www.fondazionevalsecchi.org; e-mail: [email protected]).
A press release is available at: https://www.policlinicogemelli.it/news-eventi/il-gemelli-lancia-nab-pipac-studio-per-pazienti-con-metastasi-peritoneali-da-tumore-del-pancreas/#:~:text=Lo%20studio%20Nab-PIPAC%20in,effettuata%20durante%20una%20laparoscopia%20diagnostica